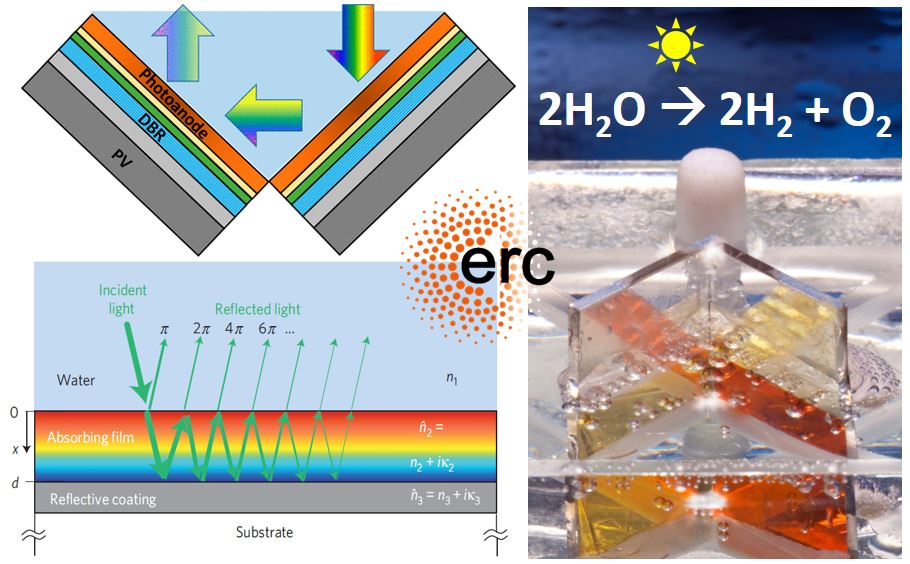
We study (photo)electrochemical materials, mostly metal oxides, and hydroxides and develop new methods, processes, and devices for energy conversion and storage. Our groundbreaking advances include:
Water photo-oxidation: We discovered that the water photo-oxidation reaction on hematite photoanodes occurs in parallel pathways with distinct metastable (Energy & Environmental Science 2022). The different pathways may interact and compete with each other to yield complex nonlinear dynamics, as we reported earlier in the presence of dilute H2O2 in alkaline electrolyte (Nature Communication 2018).
Wasted photons: We discovered that low photogeneration yield is responsible for the underperformance of hematite photoanodes for photoelectrochemical water splitting, and developed new analytical methods to extract it from spectroscopic measurements in ultrathin (Nature Materials 2021) and thin films (Energy & Environmental Science 2021).
E-TAC water splitting: High-efficiency (98.7%HHV) membraneless decoupled water splitting in a two-stage chemical – electrochemical cycle (Nature Energy 2019). This invention has led to the foundation of H2Pro, an Israeli company that develops a transformative water electrolysis technology for green hydrogen production at scale.
Decoupled water splitting: Using Ni(OH)2/NiOOH charge storage auxiliary electrodes, we decouple the hydrogen and oxygen evolution reactions and divide the electrolytic cell into separated hydrogen and oxygen cells (Nature Materials 2017). This allows centralized hydrogen production in a solar field with distributed photoelectrochemical cells that produce oxygen (Joule 2020).
Resonant light trapping in ultrathin films: Combining wave optics and ray optics in harmony to boost the light-matter interaction and photon harvesting yield of ultrathin semiconductor photo-absorbers (Nature Materials 2013).