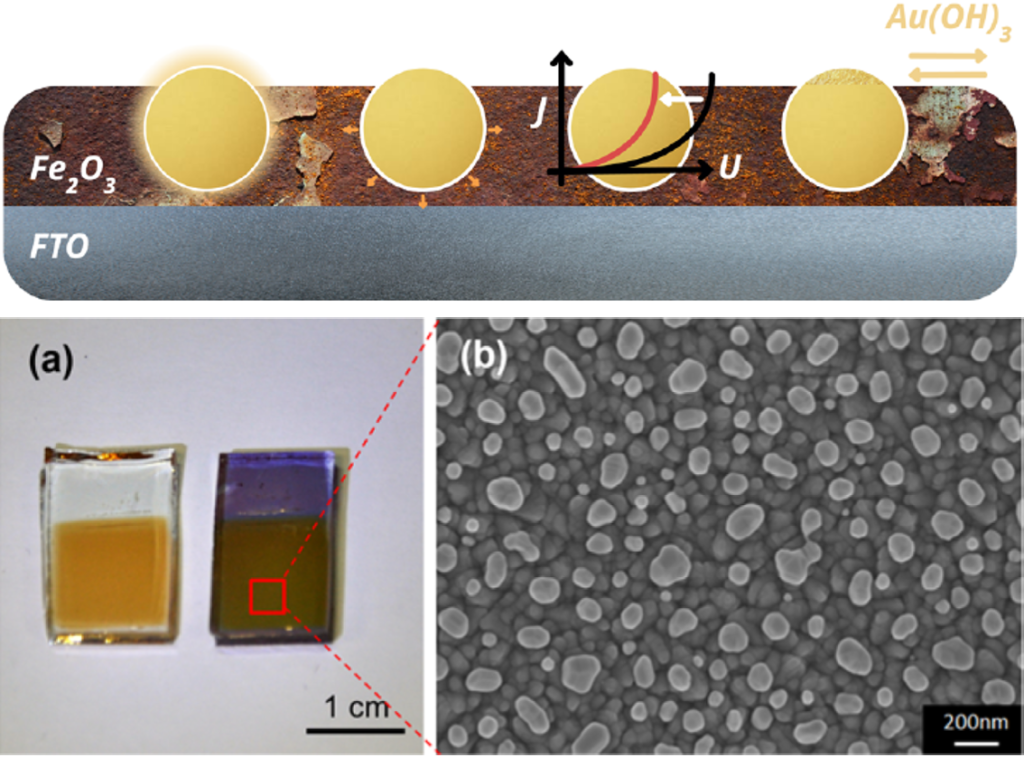
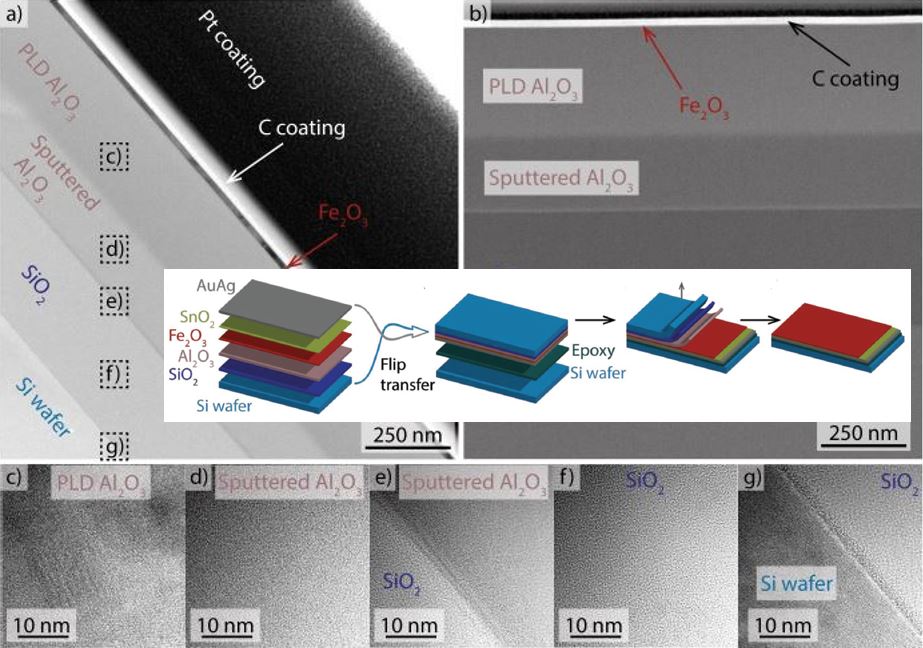
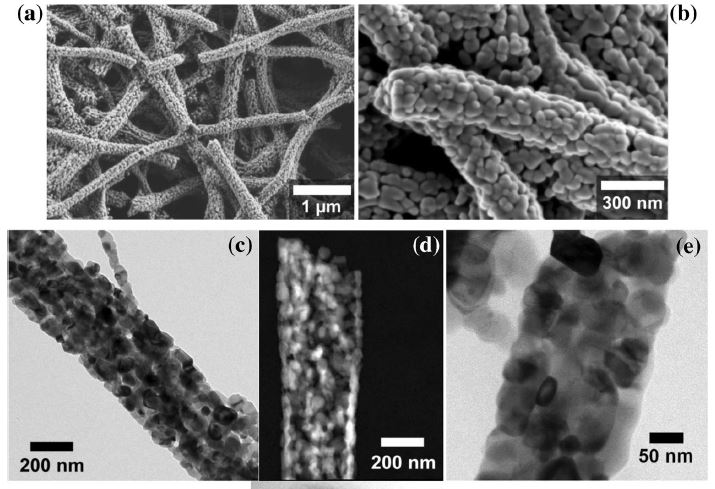
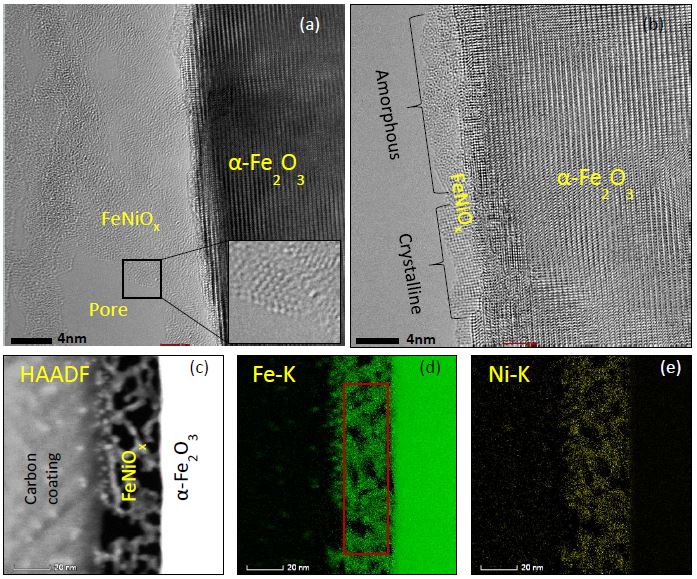
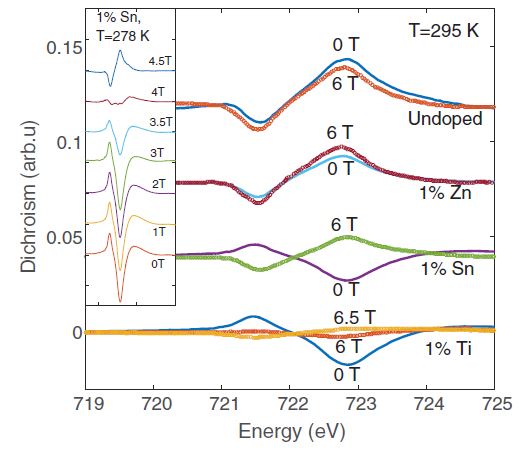
Our research aims at understating composition – structure-property relationships in electrochemical materials, mostly metal oxides and hydroxides, in order to improve their functionality in electrolytic devices for energy conversion and storage. We currently work on electrolytic water splitting to produce green hydrogen, focusing on disruptive decoupled water splitting routes, such as our recent invention of E-TAC water splitting. This breakthrough process offers potential for high efficiency water electrolysis in a membraneless cell that allows operation under high pressure and dynamic load conditions as provided by solar and wind power. One of the greatest challenges in transforming this new concept into a game changer water electrolysis technology is overcoming the capacity–rate tradeoff of the charge storage anodes. The anode in the E-TAC process divides the oxygen evolution reaction (OER, 4OH– → O2 + 2H2O + 4e–) into two stages: an electrochemical stage that charges the anode (Ni(OH2) + OH– → NiOOH + H2O + e–), followed by a chemical stage in which the charged anode reacts with water to produce oxygen and regenerates back to its initial state (4NiOOH + 2H2O → 4Ni(OH)2 + O2). By doing so, the four electron OER is modified such that it now spreads over four Ni atoms instead of one, thereby reducing the overpotential which leads the way to high efficiency in the E-TAC process. However, the anode in our process acts as a charge storage electrode where the reaction occurs in the bulk, as in batteries, rather than an inert electrocatalytic electrode where the reaction occurs at surface, as in conventional water electrolysis. As a result, the current x time product is limited by the finite charge capacity that can be reversibly charged and regenerated in the E-TAC cycles, limiting the process performance. Our goal is to understand the limiting factors and develop new materials for high-capacity and high-rate electrodes to allow long E-TAC cycles at high current density and high efficiency. In parallel, we develop a new decoupled water electrolysis process that overcomes these limitations.